Send crayfish to the Biosecurity Sciences Laboratory
If you have sick or dying crayfish that need to be submitted for examination and diagnosis by a pathologist, follow the instructions listed below.
- Select 6 or more sick crayfish typical of the problem. Similar samples will be needed from each pond or several ponds are affected.
- You may send live crayfish or preserved crayfish.
How to send live crayfish
- Crayfish should be sent in an aquaculture-standard coolite box. Keep crayfish cool and damp at all times. Use rolled-up newspapers or mesh onion bags as packing material in the coolite box to cushion the crayfish and to soak up any excess water.
- Place a sealed ice container or frozen gel pack at the base of the box. Wrap the coolant in some of the packing material to prevent crayfish coming into direct contact with the coolant.
- Place the crayfish in the box and tape the lid securely in place.
- Fill out a specimen advice sheet and tape it to the top of the box.
- Organise transportation to the laboratory, with minimal transit time.
- Contact the laboratory and advise them how and when the crayfish are coming.
How to prepare preserved crayfish
Use only live, sick crayfish for preservation.
Davidson's solution is the preferred preservative for preserving crayfish. However, if not available, 10% buffered neutral formalin may be used.
Davidson's solution
- Glacial acetic acid 115mL
- Formalin (37–40% w/v formaldehyde solution) 220mL
- 95% ethyl alcohol (ethanol) 330mL
- Tap or distilled water 335mL
If there will be a long delay in sending the preserved crayfish to the laboratory, replace the Davidson's solution with 50% ethyl alcohol (ethanol) after 24–72 hours.
10% buffered neutral formalin
- Formalin (37–40% w/v formaldehyde solution) 100mL
- Distilled or tap water 900mL
- Disodium hydrogen phosphate 6.5g
- Sodium dihydrogen phosphate 4.5g
Crayfish specimens may be held in 10% buffered neutral formalin indefinitely.
Always wear gloves and protective eye wear when handling preservatives.
How to send preserved crayfish
Use the following steps:
- Place freshwater crayfish in a cool room at below 4°C until it is insensible. A crayfish is insensible when the abdomen and tail can be easily extended or manipulated and the outer mouth parts can be moved without resistance.
- Marine crayfish should be placed into an iced saltwater slurry at 1°C, made up of 1 part ice, 3 parts saltwater for a minimum of 20 minutes or until insensible.
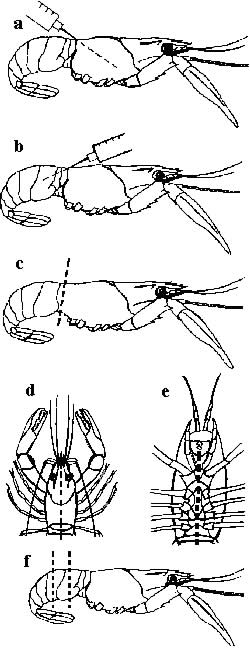
- The crayfish should be humanely killed by rapidly destroying the nerve centres. This is done by cutting through the centre line of the head and tail (splitting) (Figures D and E).
- For crayfish over 30mm long, use a syringe and a 21-gauge needle to inject the preservative (preferably Davidson's solution) into the crayfish to preserve it. Inject 1–2mL fixative into the head (Figure A) and 1–2mL into the tail (Figure B). Cut off the head (Figure C). Cut out a segment of tail (Figure F). Place all these pieces into the preservative.
- For crayfish 10–30mm long, place the whole crayfish into the fixative solution.
- Make sure there is 10 times the volume of fixative for each volume of tissue.
- When sending preserved material to the laboratory, pour off the preservative. Place a piece of paper towelling or cloth around the crayfish specimens to keep them moist and to absorb any free preservative.
- Place crayfish specimens into a plastic bag, remove excess air and seal with heavy packing tape. There should be no free fluid in the bag. Place the bag and crayfish specimens into a second plastic bag, remove excess air and seal with heavy packing tape. There should be no smell of preservative from the package.
- Label each bag if more than 1 lot of crayfish specimens is to be sent in the same box.
- Place labelled bags containing the crayfish specimens into a strong cardboard or aquaculture-standard coolite box. Add packing to prevent crayfish specimens being damaged during transport.
- Complete the specimen advice sheet, place it in an envelope and attach it to the exterior of the box containing the crayfish specimens.
- Contact the relevant Aquatic Pathologist or Veterinary Officer before sending samples to the appropriate laboratory, as listed below.
Submitting samples
Contact the Duty Pathologist before sending samples.
Routine aquatic animal samples
Submit routine aquatic animal samples for testing to:
Biosecurity Sciences Laboratory (BSL)
Health and Food Sciences Precinct
Specimen receipt (Loading Dock 12)
39 Kessels Road
COOPERS PLAINS QLD 4108
Phone: (07) 3708 8762 (Aquatic Pathologist – submission enquiries)
Further assistance
If you need further assistance, contact us online, by phone or in person.